
Chloroethane and hydrocyanic acid have trigonal pyramidal structures these molecules have three bonds running between each pair of atoms, creating an overall pyramid shape. Chlorine trifluoride has a linear molecular geometry with three unpaired electrons in its outer shll.īrmine pentafluoride has a linear structure with five unpaired electrons on its outer surface. This molecule also has two unpaired electrons. Hydrogen sulphide has a linear molecular geometry, meaning that its molecular bonds run along one straight line. But in the structure hydrogen atoms are polarised sidewise in their tetrahedral geometry. Two lone pair of electrons on the central sulfur atom is responsible for the tetrahedral nature of H2S molecular geometry. It means there are two lone pairs of electrons in the core sulfur atom. Two hydrogen atoms have no lone pairs of electrons. In the H2S electron geometry structure, the lone pairs on the central sulfur atom are two, lone pairs of electrons in the hydrogen atom have zero. Two hydrogen atoms are connected with the central sulfur atom. The lone pair of electrons in the hydrogen atom of the H2S molecule is zero. But we are considering only one connection for the calculation. This gives a total of two S-H single bond connections. Terminal hydrogen atomâs valence electron in H2S = V.EĬalculation for hydrogen atom lone pair in H2S molecule.įor instance of H2S, their terminal atoms, hydrogen, have one electron in its outermost valence shell, one S-H single bond connection. Lone pair on the terminal hydrogen atom in H2S = L.P Use the formula below to find the lone pair on the hydrogen atom of the H2S molecule. We use the following formula as given below Two fluorine atoms can share 2 of the 8 electrons resulting \ hybridization as follows:Īccording to VSEPR theory, \ hybridization corresponds to trigonal bipyramidal geometry, which is represented as follows:ĭon’t Miss: Who Is Paris Jackson’s Real Father Calculating Lone Pair Of Electrons On Hydrogen In The H2s Geometry:įinding lone pair of electrons for the terminal hydrogen atom is not similar to the central sulfur atom. There are 7 valence electrons in iodine also 1 negative charge adds one more electron thus, the total number of electrons surrounding iodine will be 8 as follows: Since fluorine is more electronegative than iodine, iodine will be at the center. In \, both iodine and fluorine belongs to the halogen family and have 7 electrons in the outermost valence shell. Electrons that are not taking part in bonding are represented as dots and are known as lone pairs of that atom.
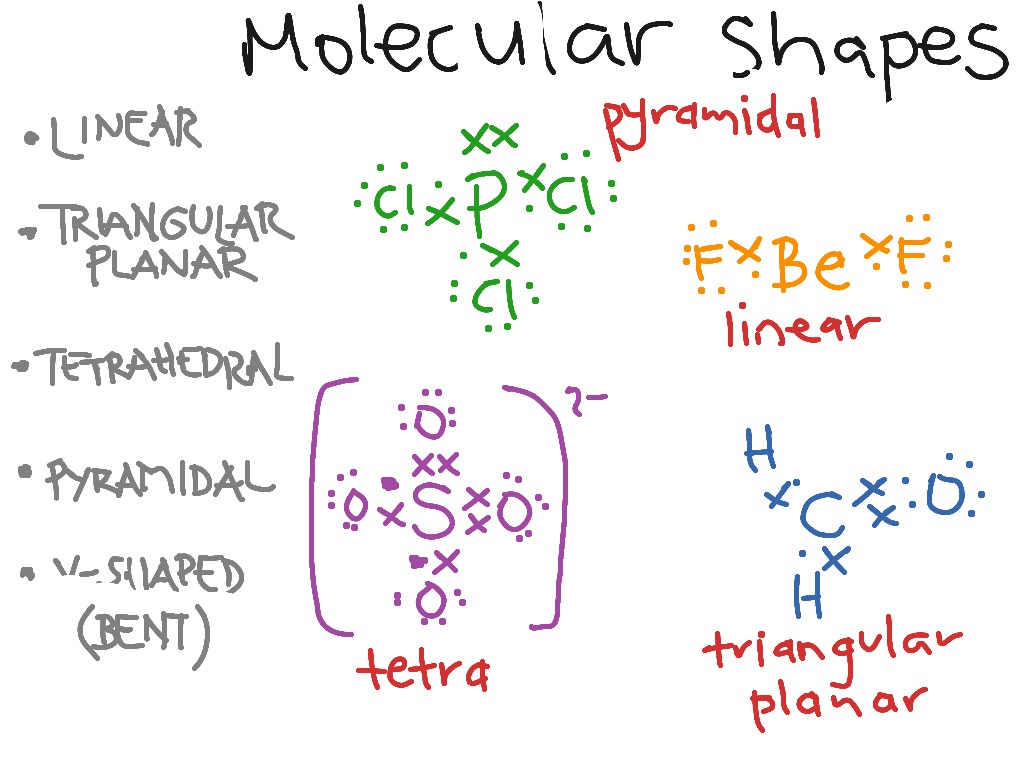

Distribute electrons in such a way that the octet of every atom is complete.Ĥ. Count the total number of valance electrons by taking the sum of each atom’s valence electron.ģ. First, draw the molecule’s skeleton, keeping more electronegative atoms at the terminal position and less electronegative at the center.Ģ. There are certain rules for writing Lewis structure of a molecule. Both bond pairs and lone pairs are contributing to determining the geometry of molecules. This is used to tell the geometry molecules taking electron pairs surrounding their central atoms into consideration. The problem is based on the concepts of VSEPR theory and Lewis structure. Molecular Geometry Of If2^ Expert Solution As a result, the charge distribution across the molecule becomes non-uniform. The H2SO4 molecule is polar in nature because of the bent H-O-S bonds which are present in it. Hydrogen and Oxygen form a linear bond with an angle of 180 degrees. Hence, its structure is classified as a tetrahedron with 109.5 degrees between the Sulfur and the four Oxygen atoms. In the H2SO4 molecule, Sulfur being the central atom has four paired electrons and 0 unpaired electrons. The electronegativity of atoms and the type of bond formation determine whether a molecule has polar or nonpolar nature. The electronic configuration including lone pairs gives the most stable shape to the molecule.
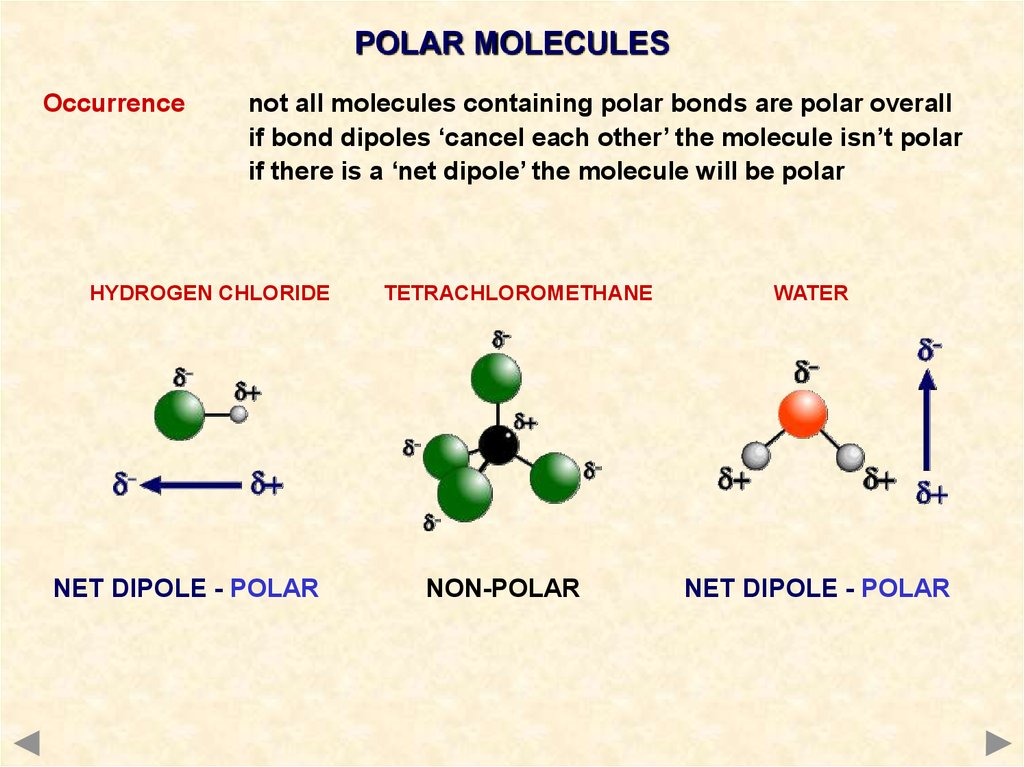
The molecular shape of a molecule and its polarity are dependent on the atoms involved in its formation. the electron pairs move to maximum angle apart from each other and become stable.Īlso, electron geometry for H2S is tetrahedral because 4 electrons which make 2 lone pairs around a sulfur atom are arranged in a tetrahedral geometry. Hence, the final shape or molecular geometry of H2S appears like a bent structure or V-shaped.Īccording to the VSEPR theory, to reduce the repulsion. This is because the two lone pair electrons on sulfur central atom repel each other as well as adjacent bonded pair electrons, as a result, these electron pair takes the position where the repulsion becomes minimum and they attains the stability. H2S Molecular Geometry / Shape and Bond Angles (Note: precise bond angle is 92.1 degrees.)


 0 kommentar(er)
0 kommentar(er)
